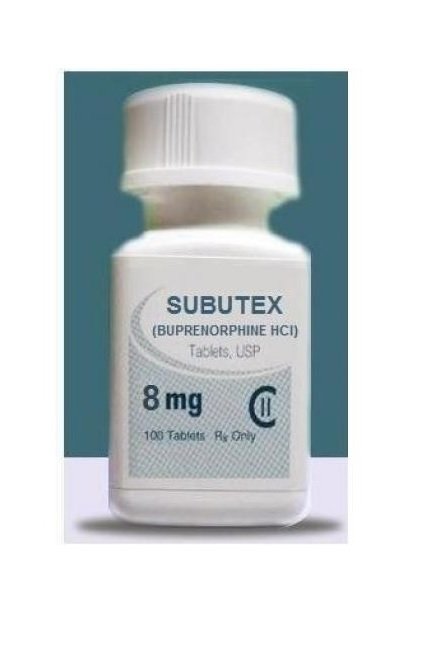
SUBUTEX (buprenorphine) sublingual tablet is an uncoated oval white flat bevelled edged tablet, debossed with an alphanumeric word identifying the product and strength on one side. It contains buprenorphine HCl, a partial agonist at the mu-opioid receptor, and is available in two dosage strengths, 2 mg buprenorphine and 8 mg buprenorphine (as the free base, equivalent to 2.16 mg buprenorphine hydrochloride USP and 8.64 mg buprenorphine hydrochloride USP). Each tablet also contains lactose, mannitol, cornstarch, povidone K30, citric acid, sodium citrate and magnesium stearate.
Chemically, buprenorphine HCl is (2S)-2-[17-Cyclopropylmethyl-4,5α-epoxy-3-hydroxy-6-methoxy6α,14-ethano-14α-morphinan-7α-yl]-3,3-dimethylbutan-2-ol hydrochloride. It has the following chemical structure. Buprenorphine HCl has the molecular formula C29 H41 NO4 • HCl and the molecular weight is 504.10. It is a white or off-white crystalline powder, sparingly soluble in water, freely soluble in methanol, soluble in alcohol and practically insoluble in cyclohexane. SUBUTEX is indicated for the treatment of opioid dependence and is preferred for induction. SUBUTEX should be used as part of a complete treatment plan to include counseling and psychosocial support.
Under the Drug Addiction Treatment Act (DATA) codified at 21 U.S.C. 823(g), prescription use of this product in the treatment of opioid dependence is limited to healthcare providers who meet certain qualifying requirements, and who have notified the Secretary of Health and Human Services (HHS) of their intent to prescribe this product for the treatment of opioid dependence and have been assigned a unique identification number that must be included on every prescription.
SUBUTEX is administered sublingually as a single daily dose. SUBUTEX does not contain naloxone and is preferred for use only during induction. Following induction, SUBOXONE sublingual film or SUBOXONE sublingual tablet is preferred due to the presence of naloxone when clinical use includes unsupervised administration. The use of SUBUTEX for unsupervised administration should be limited to those patients who cannot tolerate SUBOXONE sublingual film or SUBOXONE sublingual tablet; for example, those patients who have been shown to be hypersensitive to naloxone.
Medication should be prescribed in consideration of the frequency of visits. Provision of multiple refills is not advised early in treatment or without appropriate patient follow-up visits.
Prior to induction, consideration should be given to the type of opioid dependence (i.e., long-or short-acting opioid products), the time since last opioid use, and the degree or level of opioid dependence.
At treatment initiation, the first dose of SUBUTEX should be administered only when objective and clear signs of moderate opioid withdrawal appear, and not less than 4 hours after the patient last used an opioid.
It is recommended that an adequate treatment dose, titrated to clinical effectiveness, should be achieved as rapidly as possible. The dosing on the initial day of treatment may be given in 2 mg to 4 mg increments if preferred. In some studies, gradual induction over several days led to a high rate of dropout of buprenorphine patients during the induction period.
In a one-month study, patients received 8 mg of SUBUTEX on Day 1 and 16 mg SUBUTEX on Day 2. From Day 3 onward, patients received either SUBOXONE sublingual tablet or SUBUTEX at the same buprenorphine dose as Day 2 based on their assigned treatment. Induction in the studies of buprenorphine solution was accomplished over 3-4 days, depending on the target dose.
Patients dependent upon methadone or other long-acting opioid products may be more susceptible to precipitated and prolonged withdrawal during induction than those on short-acting opioid products; therefore, the first dose of SUBUTEX should only be administered when objective and clear signs of moderate opioid withdrawal appear, and generally not less than 24 hours after the patient last used a long-acting opioid product.
There is little controlled experience with the transfer of methadone-maintained patients to buprenorphine. Available evidence suggests that withdrawal signs and symptoms are possible during induction onto buprenorphine. Withdrawal appears more likely in patients maintained on higher doses of methadone (>30 mg) and when the first buprenorphine dose is administered shortly after the last methadone dose.

















Reviews
There are no reviews yet.